Embryo Cells Sort by Softness
Stem cells in an early-stage mammalian embryo develop into two types that need to segregate. The cells’ mechanical properties are thought to influence this segregation in some way, and experiments now suggest that the stiffnesses of the two cell types are key [1]. The results may improve researchers’ understanding of embryo development and might also be applicable to other processes, such as the progression of cancer tumors.
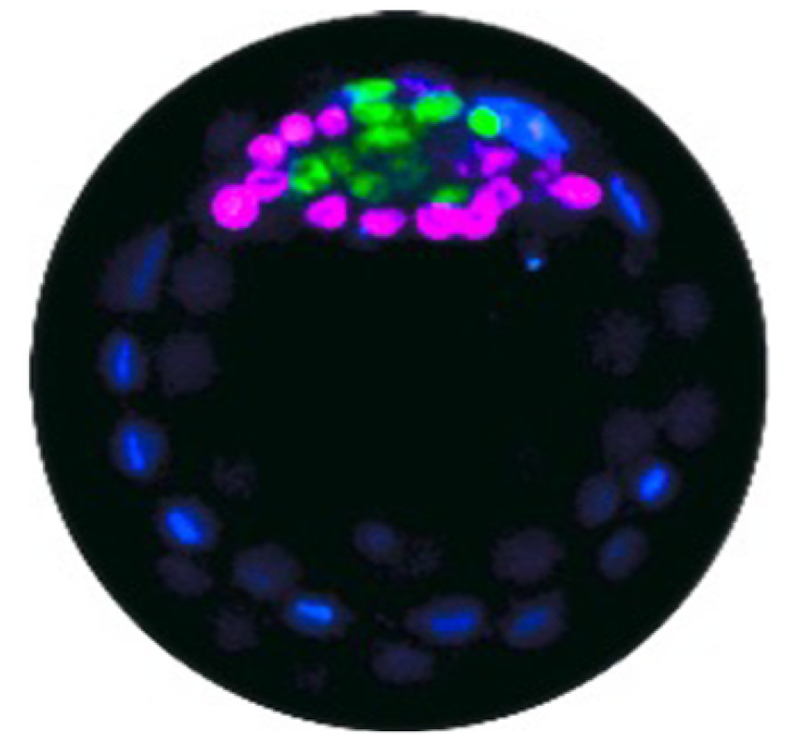
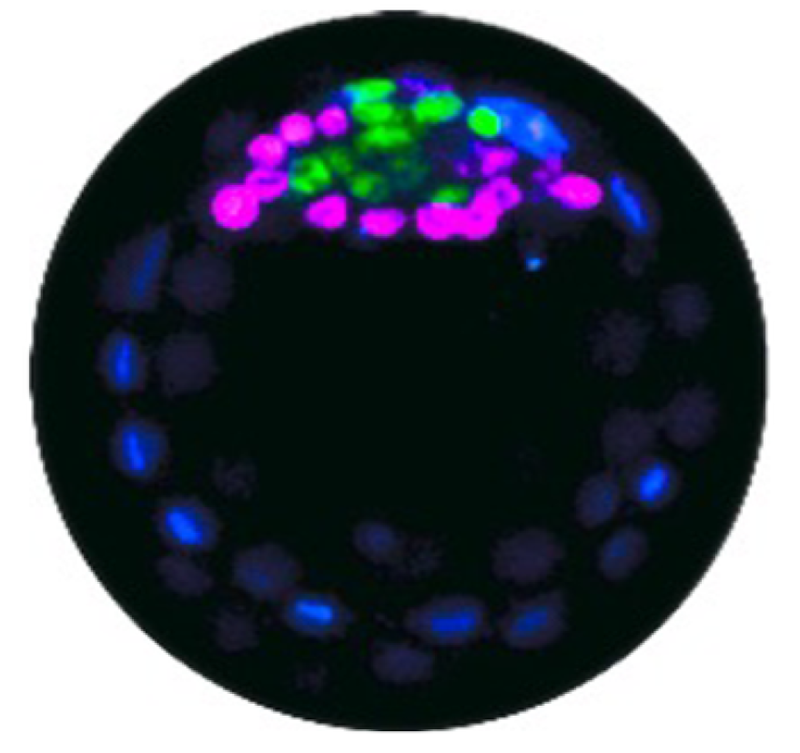
Early on, an embryo forms a blastocyst, a spherical shell in which a clump of stem cells called the inner cell mass sticks to a region of the inner wall. These cells develop into two types: epiblast (EPI) cells, which will ultimately form the fetus, and primitive endoderm (PrE) cells, which form the yolk sac and other supporting tissues. The two types emerge in a random arrangement in the inner cell mass, but they then need to segregate to form a layer of PrE cells covering the blob of EPI cells attached to the blastocyst wall.
It was long believed that the segregation was driven by differences in adhesion of the two cell types, causing a like-with-like attraction between cells, analogous to phase separation in liquids and alloys. But that idea doesn’t seem to work because EPI and PrE cells don’t have significantly different adhesion [2]. Another proposed explanation is that one cell type has a more irregular and fluctuating membrane surface [3].
Christine Ritter and colleagues at the University of Copenhagen, Denmark, offer another possibility. They investigated the behavior of mouse embryonic stem cells when they are grown in a dish through the early developmental stages. At the stage studied by the researchers, the cells have just begun the transition to—are “primed toward”—the EPI and PrE types and are called primed EPI (pEPI) and primed PrE (pPrE) cells.
Ritter and colleagues measured the stiffnesses (elasticities) of individual cells by observing droplet-like clusters of lipids called granules that the cells contain. The researchers used optical tweezers to keep a single granule confined and then watched it undergo Brownian motion. From this jiggling they could calculate the internal cellular fluid’s viscosity and thus the cell elasticity (technically, the Young’s modulus, a measure of how much a material deforms for a given amount of stress).
They found that pPrE cells are significantly stiffer (more solid-like) than pEPI cells. Using a high-resolution microscopy technique, the team deduced that this elasticity difference is attributable to the larger number of stiff filaments, made from the protein actin, in the pPrE cells.
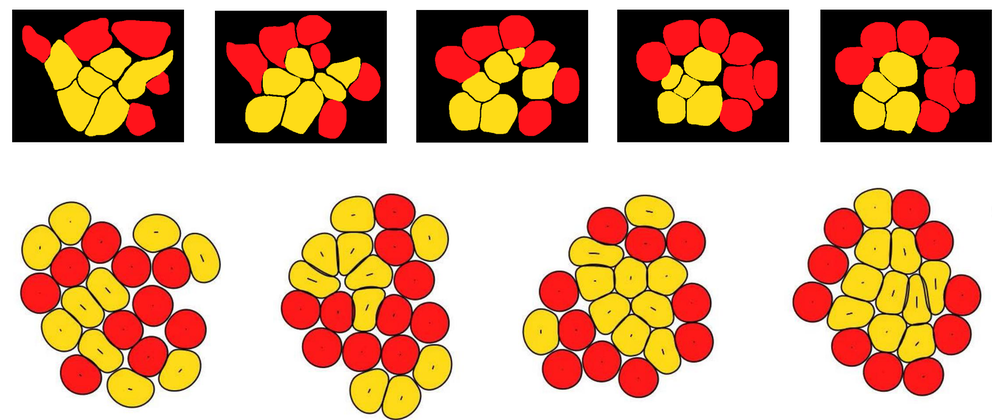
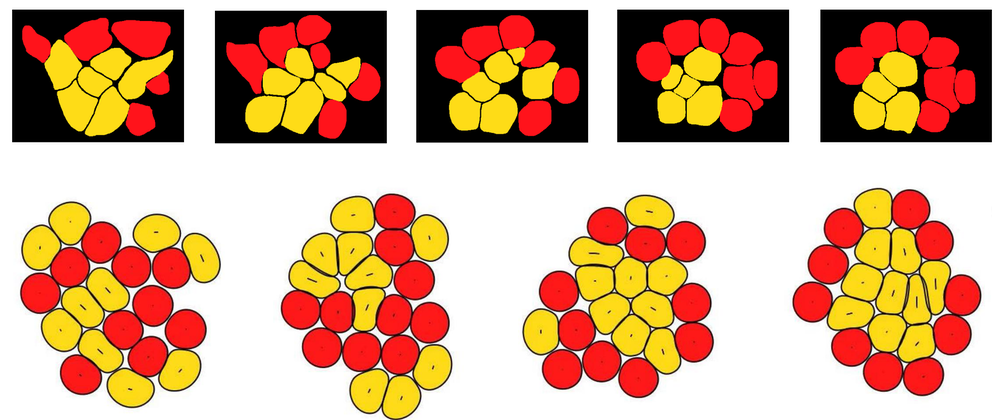
To determine whether this difference in elasticity could induce the cell sorting that the researchers observed in their cell cultures over the course of a few hours, they carried out two- and three-dimensional simulations of conglomerates of stiff and soft deformable cells. The simulations incorporated the fact that the cells experience internal stresses resulting from the constant movements of an internal web of protein filaments. According to the simulations, this “active” stress causes the stiffer pPrE cells to gradually migrate to a layer on the outside of a central mass of the softer pEPI cells. This segregation occurs because the softer cells can wiggle between the stiffer ones. “Soft cells deform more easily, enabling more frequent neighbor exchanges,” says team member Amin Doostmohammadi. “This gives them a fluid-like behavior, allowing them to infiltrate and cluster within regions of stiffer, more solid-like cells.”
“The idea of cell elasticity as a trigger for cell segregation is quite new,” says biophysicist Benoît Ladoux of the Max Planck Center for Physics and Medicine in Germany. “The idea that mechanical properties like deformability and elasticity influence sorting and segregation could potentially point to a more general principle,” he adds. For example, it may be relevant to the behavior of cancerous tissues. But he says that it is not yet clear whether the proposed mechanism is the correct one, given the other possibilities reported previously. In fact, Ritter and colleagues suspect that in real embryos there are several mechanical factors at play and that the importance of each depends on the embryo’s developmental stage.
Jenny Nichols, a developmental biologist at the University of Edinburgh in the UK, cautions that cell cultures may not be directly comparable with real embryos. And testing the proposed mechanism won’t be easy, she says, because “it would be extremely challenging to find a believable way to check the properties of cells in vivo without breaking the embryo apart.” But Doostmohammadi counters that, given the combination of experiments and modeling, “we’re confident that the mechanism we propose can operate in vivo, either as a dominant driver or in combination with other mechanisms.”
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- C. M. Ritter et al., “Differential elasticity affects lineage segregation of embryonic stem cells,” Phys. Rev. Lett. 134, 168401 (2025).
- K. Filimonow et al., “No evidence of involvement of E-cadherin in cell fate specification or the segregation of Epi and PrE in mouse blastocysts,” PLoS ONE 14, e0212109 (2019).
- A. Yanagida et al., “Cell surface fluctuations regulate early embryonic lineage sorting,” Cell 185, 1258 (2022).